
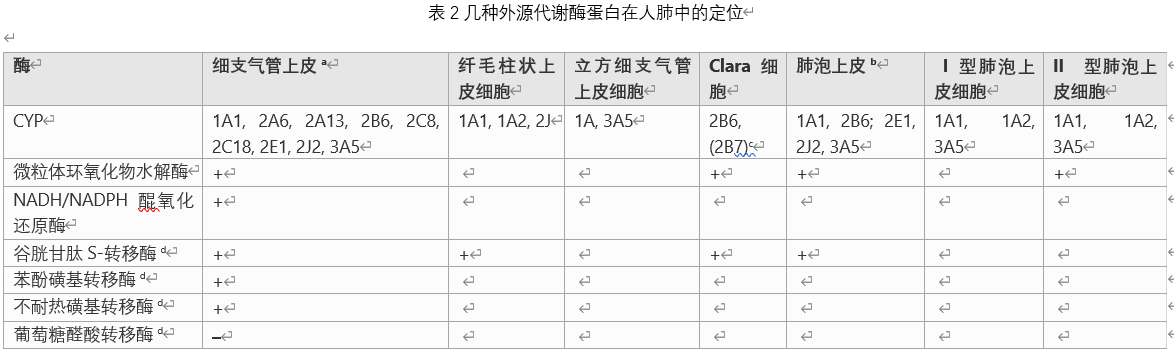
Reviewing the data collected from the review and the summary of Table 11 at the end of the review, we can see that the choice of the appropriate system is different, as long as the metabolism of the compound under consideration involves different enzymes. Since the different properties of compounds may depend on the degree of removal of different metabolites or parent compounds, even specific compounds may require different systems to study different properties. The lungs of mammals are highly complex organs with many compartments and more than 40 different cell types. The data collected in this paper indicate that the patterns of exogenous metabolic enzymes in these different cell compartments and cell types are different. Most of the information currently available to identify the relative suitability of a single experimental animal species or human in vitro model in simulating human lung conditions is based on whole organ lungs, such as lung homogenate and partial lungs. Obtaining changes in the level and/or activity of these enzymes during carcinogenesis and in cancer cells, as well as polymorphisms and their effects on cancer susceptibility, are not included in this review and may become the future The theme of the review.
(1) Certain lung metabolic enzymes in the lungs, such as cytochrome P450 (CYP), have very low activity and are difficult to accurately measure.
(2) Most of the activities cited in this comment and/or table are from the original publication, which does not state whether the given rate has been correctly tested under certain linear conditions. Therefore, not all provided values can be compared. Therefore, it is wise to use the values quoted in this review to represent only high, medium, low or unobserved activities. In most cases of "zero activity", the author does not give a detection limit, so similar considerations apply to low activity and inactivity, and in fact the number of low activity given by one author may be lower than others The value given by the author. There is no activity. The exogenous metabolic enzyme family/superfamily was briefly introduced when it first appeared. Rat lung exogenous metabolic enzymes: Cytochrome P450: Cytochrome P450 is the main exogenous metabolic oxidoreductase in mammalian liver and some extrahepatic tissues.
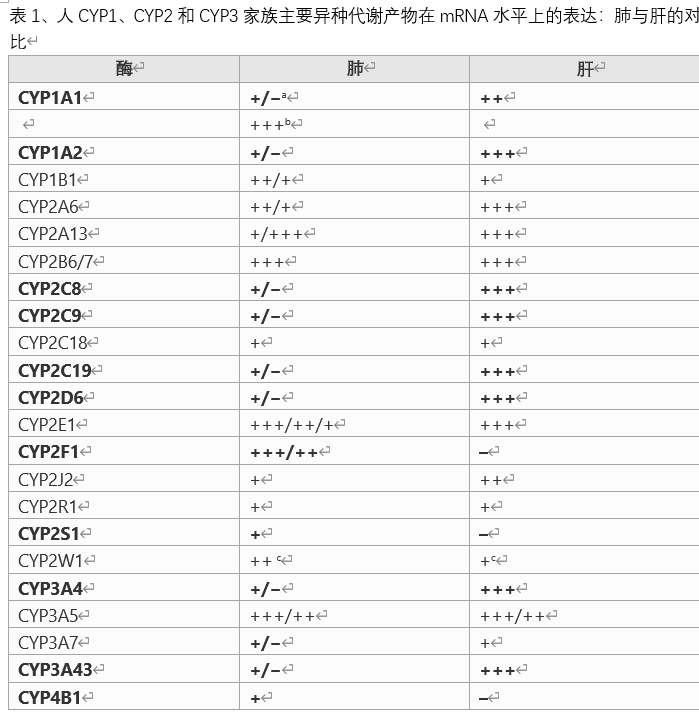
CYP reductase: Zhu et al. (1985) CYP4A mRNA was found in rat pulmonary artery endothelial cells and smooth muscle cells, bronchial epithelial cells and smooth muscle cells, type I epithelial cells and macrophages. Hellmold (1993) et al. reported the expression of CYP4A2 and CYP4A8 in rat lungs. Zeldin et al. (1996) CYP2J3 mRNA was found in rat lungs. Lake etc. (2003) Induced CYP1A1 mRNA (up to 8.3 times) by exposure to BNF (β-naphthoflavone), aroclor1254 or BP in precisely cut rat lung sections. Gate et al. (2006) reported that after rats were exposed to asphalt smoke, CYP1A1 and 1B1 in rat lungs increased significantly (26-2203 times), and CYP2F2 mRNA levels decreased significantly (2.4 times). done. Non-CYP oxidoreductase flavin-dependent monooxygenase (FMO): FMO occurs in the liver of mammals and appears to be an important heterologous metabolic oxidoreductase in extrahepatic tissues. The latter is more important than CYP. This is more important. However, their substrate specificity is limited to the conversion of nitrogen and sulfur atoms in the soft nucleophilic center, especially heterogeneous biomolecules, into N-oxide and S-oxide. Sukumaran (2011) et al. The presence of FMO2 and FMO3 mRNA in rat lungs and their circadian rhythm changes are reported. AD(P)H: Quinone oxidoreductase (NQO): NQO avoids semiquinone free radical as its main metabolite through direct two-electron reduction, thereby preventing quinone toxicity. QO converts carbonyl functional groups into hydroxyl functional groups, and is usually used as a substrate for glucuronyl transferase and/or sulfotransferase binding to promote excretion. ADH or NADPH can be used as the reducing equivalent. Gate (2006) et al. reported a significant increase (6.7-fold) of NQO1 mRNA in the lungs of rats exposed to asphalt smoke. Alcohol dehydrogenase (ADH): ADH converts alcohol into aldehydes or ketones. Ethanol is mainly converted to acetaldehyde through ADH, which can cause acute toxicity of ethanol. AD serves as an electron acceptor. Aldehyde dehydrogenase (ALDH): ALDH converts aldehydes into corresponding carboxylic acids. This step usually represents detoxification. Aldehyde oxidase (AO): AO catalyzes the conversion of aldehydes to carboxylic acids by simultaneously forming hydrogen peroxide. Use molecular oxygen as an electron acceptor. Glutathione S-transferase (GST): GST converts electrophilic foreign substances into glutathione (GSH) conjugates. It is usually detox, but in some cases it is toxic. Glucosyltransferase: UGT converts exogenous compounds with appropriate substituents (mainly hydroxyl, sometimes amino, sulfhydryl or carboxyl) into glucuronic acid. This is usually a detoxification, but in some cases it can cause toxicity (such as "acyl transfer"). Rabbit lung exogenous metabolic enzyme: Cytochrome P450: The expression of CYP4B1 mRNA in rabbit lung tissue is significantly higher than that in liver and other tissues.
Expression of CYP and related proteins: Serabjit Singh et al. (1980) observed CYP reductase protein in rabbit lung Clara cells. The localization of CYP reductase in rabbit lung carat cells is higher than that in type II alveolar macrophages. CYP reductase protein is not expressed in rabbit type I lung cells and endothelial cells. Although CYP1A1 and CYP1A2 proteins were not detected in rabbit lung tissue, TCDD can induce the expression of CYP1A1 and CYP1A2 proteins in bronchus, bronchial epithelium, pulmonary artery, vein and capillary endothelial cells. CYP1A1 is low in rabbit alveolar macrophages. CYP2B4 was observed in rabbit Clara cells and type II lung cells, bronchial and bronchial epithelium, and type I lung cells.
The relative content of CYP2B4 is higher in Clara cells, while the relative content of CYP2B4 is lower in bronchial and bronchiolar epithelial cells. CYP4B1 has the highest content in Clara cells, bronchial ciliated cells, bronchial ciliated cells, type II lung cells and capillary endothelial cells, but not in type II lung cells. Non-CYP oxidoreductase: Flavin-dependent monooxygenase (FMO), aldehyde ketone reductase (AKR). Hydrolase: Epoxyhydrolase (EH): Microsomal epoxyhydrolase (mEH, also known as EPHX1), soluble epoxyhydrolase (sEH, also known as EPHX2). Golden hamster lung exogenous metabolic enzyme: cytochrome P450 (CYP): Lorenz et al. (1984) showed that golden hamster lung microsomes can effectively catalyze the broad-spectrum CYP substrate 7-ethoxycoumarin. Like rat lung microsomes, ethyl is about 5 times slower than mouse lung microsomes, but 100 times faster than human lung microsomes. For a long time, lung Clara (Club) cells in golden hamsters have been identified as the main location of CYP-dependent covalent binding. Paolini et al. (1995) reported the induction of CYP2B-dependent PROD activity in golden hamster lungs and several compounds (phenobarbital sodium, barbital sodium, cyclophosphamide). Binding enzymes: glutathione S-transferase (GST), sulfotransferase (SULT). Exogenous metabolic enzymes in guinea pig lungs: Cytochrome P450 (CYP): Bilimia, etc. (1977) Observed the AHH activity of guinea pig lung homogenate. After animals are exposed to cigarette smoke, AHH activity is significantly reduced (about 50%). Exogenous metabolizing enzymes in pig lung: Cytochrome P450 (CYP): CYP1A1 and CYP1A2 transcripts were observed in pig lung. The constitutive expression level of pig CYP2B22 (homologous to human CYP2B6 and 81% nucleotide homology) mRNA is higher than that of pig liver. CYP2D25 (homologous to human CYP2D6, 83% nucleotide homology) is expressed at low levels in pig lungs. Porcine lung tissue contains CYP3A22, 3A29 and 3A46 mRNA, but their expression levels are much lower than those in the liver. Coupling enzyme: glutathione S-transferase (GST). Extrinsic metabolic enzymes in dog lungs: Cytochrome P450 (CYP): Visser et al. (2017) observed the expression of CYP2B11 transcript in dog lung and the lowest expression of mRNA of other exogenous metabolic enzymes. The monooxygenation of several exogenous compounds catalyzed by CYP has been observed in dog lungs. Hydrolase: Epoxyhydrolase (EH). Coupling enzyme: glutathione S-transferase (GST). Exogenous metabolic enzymes in monkey lung: mar monkey: cytochrome P450 (CYP): Uehara et al. (2018) observed that the high expression of CYP2F1 transcripts in mar monkey lungs far exceeds that of any other research institute. High (liver, kidney, jejunum, brain).
Western blotting showed that mar monkey lung CYP2F1 protein cross-reacted with human CYP2F1 antibody. In mar monkey lung microsomes, the catalytic activity of several prototype CYP substrates was observed, such as coumarin, 7-ethoxycoumarin and 2-/4-biphenyl, but chlorzoxazone was not found. Cynomolgus monkey: Cytochrome P450 (CYP): Uehara et al. (2018) confirmed the high expression of CYP2F1 transcript in cynomolgus monkey lung. In the cynomolgus monkey lung microsomes, the catalytic activity of some typical CYP substrates (such as coumarin, 7-ethoxycoumarin and 2-/4-biphenyl) was observed, but no toxicity was observed. Die x.