
Nonalcoholic fatty liver disease (NAFLD, Nonalcoholic fatty liver disease)
is a disease in which too much fat is stored in the liver. This kind of fat
accumulation is not caused by heavy drinking. When heavy drinking causes liver
fat accumulation, this condition is called alcoholic liver disease. There are
two types of NAFLD: simple fatty liver and nonalcoholic steatohepatitis (NASH,
nonalcoholic steatohepatitis). NASH is one of the most important causes of liver
disease in the world. It has become the most common liver disease in developed
countries, and its incidence in developing countries is also increasing year by
year.
is called "Year of NASH" in 2019. NASH drug candidates of 4 pharmaceutical
companies have entered the phase III clinical development stage, and nearly a
hundred drugs have entered the clinical trials (Clinical trials). Up to now,
there are no approved effective NASH treatment drugs and programs. The FDA
stated in a guidance document that successful treatment of NASH, improvement in
fibrosis, or a combination of both are potential acceptable endpoints for
approved NASH therapy. Nevertheless, which endpoint and which drug proved useful
in the field first, there are still no accepted results.
The main risk factors for NASH include obesity, type II diabetes,
dyslipidemia and metabolic syndrome. In addition to liver steatosis, NASH also
includes swelling, inflammation and fibrosis. In the middle and late stages of
NASH, inflammation and liver cell damage can lead to liver fibrosis or scar
formation, which can further develop into liver cirrhosis or liver cancer. Based
on these characteristics, the development of animal models has also made great
progress in recent years, especially in Liver fibrosis, which eventually
develops to the stage of Hepatocellular carcinoma. Liver fibrosis refers to the
accumulation of large amounts of scar (fibrosis) tissue in the liver due to
repeated or long-term injury or inflammation. Although some animal studies have
shown the potential of the liver to regenerate or heal itself, once the human
liver is damaged, the liver usually does not heal. However, changes in drugs and
lifestyle can help prevent the deterioration of fibrosis, and mouse models can
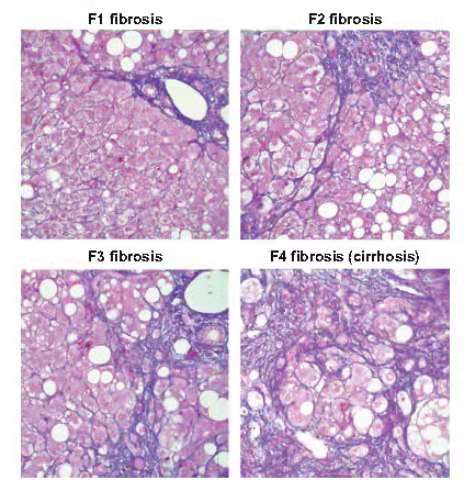
be used for preclinical evaluation. The common reference index for liver
fibrosis is the human liver fibrosis METAVIR scoring system, which divides
fibrosis into 4 levels [1]. The fibrosis stages are from F0 to F4: F0: no
fibrosis; F1: portal fibrosis without septal; F2: portal fibrosis with a small
interval; F3: large interval without cirrhosis; F4: liver cirrhosis.
NASH mouse models can be divided into 4 categories: (1) diet induction
model, (2) chemical substance induction model, (3) gene editing model, (4) the
first two, or a comprehensive model combining the three methods The advantage of
this type of model is that the pathological features of the disease model are
the most typical and comprehensive. Since fibrosis improvement is a potential
acceptable endpoint for approved NASH therapy, this article focuses on the
fibrosis model.
1. Dietary induction model
Using high-fat, high-sugar, and/or high-cholesterol feed, or MCD
(methionine choline deficient feed), can successfully induce obesity or the NASH
model of nutritional deficiency. For example, for C57BL/6 mice, using Research
Diets' D12492 feed (high-fat feed, 60% fat for energy) can induce obesity and
basically no fibrosis. Adopt D09100310 feed (NASH feed, instead of FDA prohibits
the use of feed AMLN DIET containing trans fatty acids, 40% fat for energy,
mainly palm oil, 20% fructose and 2% cholesterol), 12 to 16 weeks to induce
steatosis; 20 Inflammatory bodies are induced in ~26 weeks; fibrosis is induced
in 26~34 weeks. The characteristic of the diet-induced NASH model is that it
simulates the characteristics of obesity, type II diabetes, dyslipidemia and
metabolic syndrome, but the liver fibrosis in mice is relatively weak, and HCC
basically does not occur. In the diet induction model, adding a high proportion
of fructose, glucose and cholesterol, the liver fibrosis of mice can reach the
F2 standard (moderate moderate fibrosis), which is a preclinical mouse NASH
model that is currently widely used .
2. Chemical substance induction model
Chemical-induced liver damage and liver fibrosis are very common, and have
been used to construct mouse liver fibrosis models. The probability of such
models developing to liver cirrhosis and liver cancer is also high [2]. Commonly
used inducers are carbon tetrachloride (CCL4), thioacetamide (TAA) and
streptozotocin (STZ). CCL4 and TAA are commonly used in adult mice, and STZ is
used in newborn mice. The toxicity mechanism of CCl4 and TAA is not fully
understood, but it involves the uptake and transformation of CCl4 and TAA by
hepatocytes. The metabolites cause oxidative necrotic inflammation and the
excessive activation and proliferation of collagen-secreting cells. Using
neonatal mice with a specific background, combined with high-fat diet and STZ
induction, a "STAM" mouse model can be established, which exhibits the
characteristics of NASH at 8 weeks and fibrosis at 12 weeks, and finally nearly
100% of male rats show HCC[3].
3. Gene editing model
Genes related to metabolism, or proteins that express damage to liver
cells, researchers have developed many NASH gene editing models (see review
[2]). Here are three types of NASH gene editing models, combined with a high-fat
diet, NASH models can be obtained faster and in a higher proportion, with
obvious fibrosis characteristics. The low-density lipoprotein receptor (Ldlr)
gene family is composed of cell surface proteins involved in specific ligand
receptor-mediated endocytosis. The serum cholesterol level of Ldlr KO mice is 2
to 4 times that of wild mice. Combined with high-fat (HFD-fed) feeding LDLR ko
mice, it can induce metabolic syndrome characterized by obesity and
hypercholesterolemia, hypertriglyceridemia and insulin resistance symptoms;
similar to the human NASH model. model.
Mc4r (melanocortin 4 receptor) is a protein-coding gene. Mc4r homozygous
knockout mice weighed up to 30 g at 6 to 8 weeks, showing obvious obesity.
Combined with high-fat (HFD-fed) feeding Mc4r KO mice, it is easier to show
liver steatosis, liver fibrosis and hepatocellular carcinoma, similar to the
model characterized by the human NASH model [5].
Urokinase-type plasminogen activator (uPA) plays an important role in the
degradation of extracellular matrix. uPA is toxic to liver cells and can cause
liver damage. As early as 1992, Sandgren et al. made Alb-uPA transgenic mice to
study liver cancer. However, due to the liver toxicity of uPA, Alb-uPA mice will
show symptoms of hemolysis within 4 days of birth, and most of them will
eventually die. In 2000, Weglarz et al. made improvements [6] and made MUP-uPA
transgenic mice, using MUP enhancer/promoter to specifically express uPA in
hepatocytes for studying hepatocyte transplantation. Similar to Alb-uPA, the
expression of uPA will gradually decrease due to gene loss; the difference is
that MUP-uPA mice will not begin to express uPA until 2-4 weeks, thereby
reducing the mortality of newborn mice. A major study published in Nature in
2017 [7], MUP-uPA mice were backcrossed to the C57BL/6 background through 10
generations, fed a high-fat and high-sugar diet (WD-SW), and showed typical
results for 3 months. NASH lesions, such as liver steatosis, steatohepatitis
(including ballooning hepatocytes and Mallory-Denk bodies), after 6 to 11
months, 90% of mice develop HCC. From RNA-seq and histochemical data, it is
proved that this model is the best model reported to simulate human NASH and
HCC.
Due to the wide range of causes of NASH, it is recognized that it is a
heterogeneous disease, which also brings difficulties to the selection of
models. Relieving fibrosis as an acceptable end point for potentially approved
NASH therapy makes fibrosis indicators in animal models more important.