
Therapeutic angiogenesis has become one of the hotspots in coronary heart disease research since the 1990s. Under the background that drug therapy, interventional therapy, and surgical treatment were quite mature at that time, it was discovered that a considerable number of coronary heart disease patients still cannot benefit from existing treatment methods due to severe and diffuse coronary artery disease. Patients with sexual cardiomyopathy. Researchers at home and abroad have conducted a lot of research, using cytokines that can promote capillary angiogenesis and promote the establishment of collateral circulation for "biological bypass", trying to establish a new blood vessel network in severely ischemic myocardium. In order to carry out these studies, it is necessary to establish a chronic myocardial ischemia model that simulates the heart conditions of patients with advanced coronary heart disease.
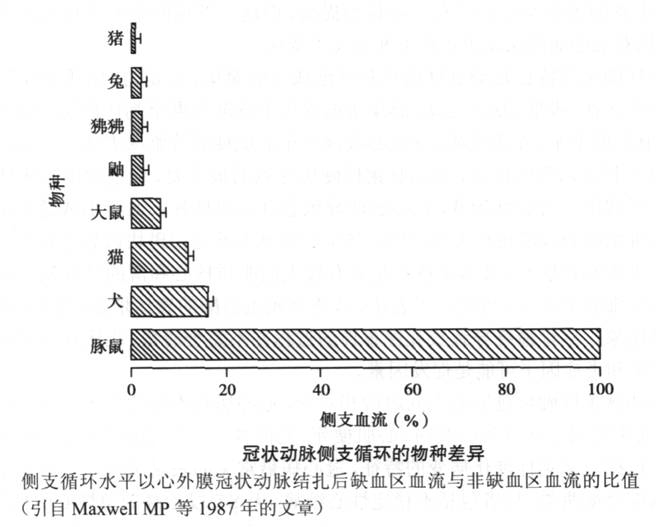
(1) Selection of Experimental Animals Maxwell et al. published an article in 1987 described the existence of collateral circulation in different types of mammals. This study shows that dogs, which are most commonly used in animal studies of myocardial ischemia, have a large number of collateral vessels when they are normal. After acute coronary occlusion, relying on the original collateral vessels can still provide up to normal levels for the myocardium. At 40% blood perfusion, this is very different from the situation of human coronary arteries. Therefore, in recent years of chronic myocardial ischemia research, pigs have become the most widely used large animal model (Figure 6-1).
The anatomical structure of the pig's coronary arteries is very similar to that of humans. There are few original collateral vessels, and the distribution of coronary arteries is close to that of humans. Most of them are right-coronary dominant. The conduction system of the pig heart is also very similar to that of humans. The ratio of heart size to body weight of miniature pigs commonly used in animal experiments with a weight of about 30 kg is about 0.005, which is also similar to that of humans. In addition, in terms of metabolism, the heart of pigs is similar to that of humans. Under normal conditions, the metabolism of the myocardium mainly uses non-esterified fatty acids as the substrate, which provides nearly 80% of the energy for the myocardium. In the case of severe myocardial ischemia, fatty acid oxidation decreases and sugar utilization increases. These anatomical and physiological factors have determined that pigs have become a more commonly used animal model in the study of myocardial ischemia, especially in the study of therapeutic angiogenesis.
At present, most laboratories no longer use ordinary domestic pigs as research materials, because these pigs have many problems, such as high fever and ventricular fibrillation under stress, inadaptability to the laboratory environment, and rapid growth. At present, miniature pigs bred by various methods are widely used in research, especially in chronic experiments, where the body weight of miniature pigs grows slowly, and can overcome the above shortcomings and is suitable for experimental research. It is recommended to use small-scale
pig. There are also many domestic breeding centers that can provide small pigs suitable for animal experiments, such as the Experimental Animal Center of China Agricultural University.
(2) Model establishment methods At present, there are mainly three types of chronic myocardial ischemia animal models that are widely used: Ameroid constrictor model (Ameroid constrictor), fixed stenosis model (fixed stenosis), and hydraulic occluder model (hydraulic occluder). ).
1. Ameroid constriction ring model (Figure 6-2 ~ Figure 6-4) This model is currently the most widely used chronic myocardial ischemia model, especially in a large number of preclinical studies of therapeutic angiogenesis. Angiogenesis-promoting proteins (such as recombinant vascular endothelial growth factor, basic fibroblast growth factor, autologous bone marrow stem cell transplantation, angiogenesis-promoting gene therapy, and myocardial laser perforation therapy and other treatment methods in animal experimental studies have used this model in large numbers .

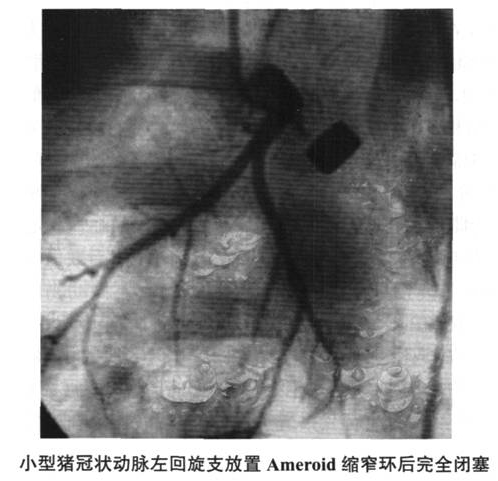
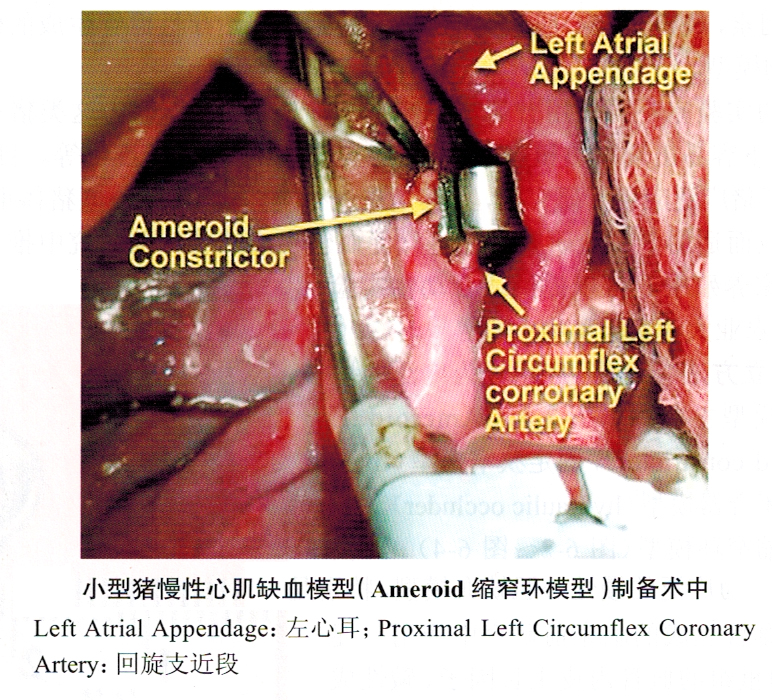
This model was first proposed by Litvak et al. in 1957. The constriction ring is made of casein hygroscopic material and covered with a steel collar. After the constriction ring is placed around the coronary artery, the absorbent material absorbs the interstitial fluid and expands inward, compressing the artery and causing complete occlusion of the coronary artery after 2 to 4 weeks. The exogenous compression caused by the constriction ring may cause damage to the vascular endothelium, causing platelet aggregation, thrombosis, and local scar formation caused by foreign body reactions. Eventually, chronic vascular occlusion may not be formed as scheduled, and unexpected acute occlusion may occur. .
Usually, for safety reasons, the constriction ring is placed on the left circumflex (LCX) of the coronary artery. This vessel is the smallest of the three main coronary arteries in pigs and supplies about 20% of the left ventricular myocardium: In addition, the original collateral vessels in the blood supply range of LCX are more abundant than the anterior descending artery and right coronary artery. Even so, the possibility of sudden death due to postoperative ventricular fibrillation or large-area myocardial infarction still exists. In the study that reported animal mortality, postoperative sudden death due to ventricular fibrillation or large-area myocardial infarction accounted for the cause of death in experimental animals. 30%. Most sudden deaths related to ischemia occur in the final occlusion of the constriction ring. As mentioned above, unlike dogs, pigs have very few original coronary collateral vessels. Therefore, they have poor tolerance to acute coronary occlusion, which can cause large-scale myocardial infarction and lead to death. Acute occlusion of porcine LCX will cause 75% of myocardial necrosis in the corresponding area and lead to 35% mortality, while the corresponding proportions in dogs are only 50% and 13%. Of course, the slow occlusion of blood vessels usually occurs after the placement of the constriction ring. At this time, the collateral circulation will gradually be established, which can prevent or limit the occurrence of myocardial infarction. Because the rate of occlusion of the constriction ring in the body is not uniform, the reported rates of myocardial infarction in ischemic areas vary greatly. O'Konski et al. observed that after placing the constriction ring on the LCX of pigs, the proportion of infarction in the ischemic area after 3 weeks was 37%±36%. Myocardial infarction mainly occurs under the endocardium. It can be seen that the scope of myocardial infarction varies greatly among different animals, and the proportion of myocardial infarction in the ischemic area is 5% to 100%. In one of the subgroups, the animals were given aspirin during the experiment, and the myocardial infarction range of this group tended to shrink (17%±6% of the ischemic myocardium). In another follow-up study reported by the same laboratory, the proportion of myocardial infarction in the LCX supply range was only 5%±1%, which the author believes is related to the reduction of coronary operations during surgery. Other studies have reported that the incidence of myocardial infarction in the ischemic area of the LCX supply range is between 6% and 13%.
Among the existing animal models, the Ameroid constriction ring model is the most widely used. At present, the understanding of coronary collateral circulation is mostly obtained from the research using this model. In order to determine the applicability of the model, many studies have been conducted on changes in local myocardial blood perfusion and myocardial function after LCX occlusion. O'Konski et al. used radioactive microspheres [in experimental studies, it is the gold standard for measuring local myocardial blood flow (MBF), which will be detailed in the third section "Introduction to animal models"]. Three weeks after the constriction, the local full-thickness MBF of the myocardium within the LCX supply range is no different from the left ventricular area without ischemia; however, under load, the local full-thickness MBF of the myocardium in this range decreases by 42% compared with the control. It suggests that the coronary blood flow reserve is impaired. A large number of other studies using radioactive microspheres to measure MBF have also consistently found that after placing the constriction ring for 3 to 4 weeks, the perfusion of the myocardium in the LCX supply range in the resting state is normal, while the MBF in the ischemic area under load Lower than non-ischemic myocardium. Some researchers measured the blood flow of the endocardium, the middle myocardium and the epicardium after the LCX constriction ring was placed. They found that the LCX supply range in the resting state at 16 weeks after the operation was not significantly different from the normal area. In this case, the blood flow of each layer of myocardium within the supply range of LCX is significantly reduced compared with non-ischemic myocardium. Goerge et al. found that when adenosine was administered to expand the coronary collateral circulation at 4 weeks after surgery, the blood flow provided by the collateral vessels was about 20% of the normal maximum blood flow, and this level could rise to 50%-60% after 8 weeks. %, but did not increase until 26 weeks after surgery. In general, the reduction in myocardial blood perfusion caused by this model only occurs under stress.
In addition to the direct measurement of blood perfusion, the researchers also used sonar micro-measurement to measure the rate of local wall thickening to reflect the function of the local myocardium. Shen and Vatner conducted a very detailed study. After the LCX constriction ring was placed, the local myocardial function was measured with ultrasonic crystals every day. They found that the systolic wall thickening rate decreased peak (56%±6 of baseline). %) After (20±3) days after surgery, it gradually returned to normal afterwards. By (34±2) days, the rate of local ventricular wall thickening did not differ from the baseline. They also pointed out that the difference in the peak time of the decline of the ventricular wall systolic function also indicates the difference in the occlusion time of the constriction ring, and the restoration of the local wall function is important for the success of the model. A large number of other studies have also shown that in the resting state, because the blood flow of the myocardium does not decrease, after 3 weeks after the placement of the constriction ring, the local wall thickening rate of the LCX supply area is similar to that of the control area; while the LCX supply area under load The function of the internal local ventricular wall was significantly lower than that of the baseline and control areas. These changes remained stable for at least 16 weeks after surgery. Therefore, from the perspective of the level of myocardial function, the Ameroid constriction ring model only exhibits myocardial contractile function decline due to ischemia under load.
White et al. observed in detail the morphology and function of the collateral vessels formed after the placement of the LCX constriction ring. Three weeks after the placement of the constriction ring, the number of collateral vessels from the other two branches of the coronary artery (inter-coronary artery) and blood vessels outside the heart (extracardiac) such as bronchial artery and internal mammary artery increased significantly. The diameter of inter-coronary collateral vessels is mostly 20-60 μm, and the thickness of the middle layer is 50% to 70% of normal arterioles (the smooth muscle in the vessel wall is obviously less), while the diameter of the collateral vessels outside the heart is larger and the wall Thicker (the thickness of the middle layer is 80% of normal). The number and size of collateral vessels will increase significantly from 3 to 8 weeks after surgery, and remain stable until 16 weeks after surgery. The inter-coronary collateral circulation is evenly distributed in the endocardium and middle myocardium, and is denser around the posterior papillary muscles, while the extracardiac collateral circulation is mainly distributed in the epicardium. The number of collateral circulation outside the heart is less than that of the inter-coronary collateral circulation. Compared with the original collateral vessels before coronary occlusion, these collateral vessels can increase collateral-related blood flow by 14 times, and the blood flow provided by the collateral circulation outside the center accounts for about 30%. DNA labeling studies on endothelial cells and smooth muscle cells showed that 2 to 3 weeks after placement of the constriction ring, the labeling index of endothelial cells and smooth muscle cells increased by 50 to 70 times, which was consistent with the establishment of collateral circulation. The corresponding labeling index will return to the baseline level at 8 weeks, indicating that there is a significant proliferation of endothelial cells and smooth muscle cells in this process. The collateral circulation of dogs is a "mature" blood vessel with a normal number of smooth muscle cells in the middle layer. After the constriction ring is placed, the collateral circulation can restore normal blood perfusion and can still meet the blood supply even under load. need. However, pigs are different. The collateral vessels of pigs lack smooth muscle cells, and the response to vasodilators and load is uncertain, so there is limited collateral circulation. This explains why the pig Ameroid model can be induced under load conditions. Ischemia.
Compared with other chronic myocardial ischemia models, the operation of the Ameroid constriction ring model is relatively simple, which is its main advantage. However, this model also has its inherent limitations. First of all, from the model establishment process, the speed and degree of coronary occlusion in this model cannot be precisely controlled. Sometimes the occlusion is too early, and sometimes it cannot be completely occluded. This may lead to significant individual differences in the proportion of myocardial necrosis in ischemic areas, and also cause deaths in animals whose blood vessels are occluded too fast, resulting in up to 30% mortality in some studies. Secondly, as mentioned earlier, although the original collateral circulation is very small, after the placement of the vascular constriction ring and the coronary artery occlusion, the pig’s heart can still form collateral vessels quickly, and after 3 to 7 weeks The blood flow and local myocardial function at rest return to normal levels. Only when the myocardial oxygen consumption increases, such as exercise load, will the reduction of myocardial blood flow and the impairment of the local myocardial function of the left ventricle occur. Therefore, this model It is actually a model of stress-induced ischemia. In patients with advanced coronary heart disease targeted by the Institute of Therapeutic Angiogenesis, a considerable number of patients have myocardial ischemia at rest and myocardial hibernation due to long-term chronic ischemia, which cannot be simulated by the Ameroid constriction ring model. Yes, this may affect the results of research conducted using this model. In addition, since this model simulates stress-induced ischemia, all studies using this model must evaluate the myocardial blood perfusion and function under stress in order to get accurate conclusions, which undoubtedly increases the research process. Complexity has also significantly increased the cost of research.
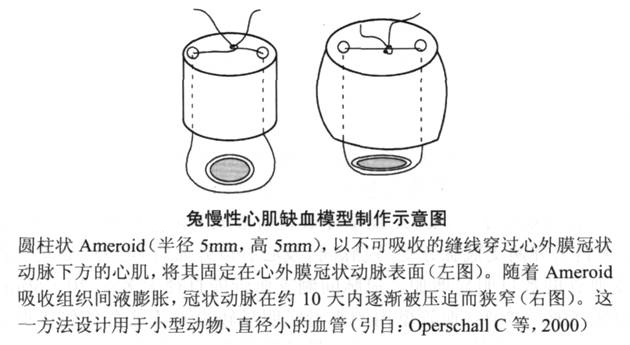
In addition to the application of Ameroid constriction rings in large animals such as pigs and dogs, some researchers have established a similar rabbit model of chronic myocardial ischemia. Due to the small size of the rabbit heart, it is impossible to use the constriction ring, so they made the constriction ring filler into a small compression piece, threaded it and fixed it above the rabbit coronary artery, and achieved chronic occlusion of the coronary artery through the expansion of the filler ( Figure 6-5).
2. Fixed stenosis model Unlike the Ameroid constriction ring model, the fixed stenosis model has never been used in the study of pro-angiogenesis therapy, but has been widely used to study the pathophysiological changes of hibernating myocardium. Different from the Ameroid model, when the fixed stenosis model is used, the severe fixed stenosis of the coronary artery will cause the MBF of the corresponding blood supply area to decrease significantly at rest. The reason for this difference may be due to less collateral circulation formation when the degree of vascular stenosis is less. In previous studies conducted in pigs, it was indeed found that no significant collateral circulation would be formed without complete coronary occlusion. This is similar to the situation in humans, who usually only develop significant collateral circulation when the epicardial coronary artery stenosis exceeds 90%, although there are differences between individuals.
The method of establishing a fixed stenosis model is usually to tie the coronary artery to a fixed size by some method. Chen et al. used a silk knot to surround the proximal end of the anterior descending branch to reduce the outer diameter of the artery and reduce the resting blood flow in the stenotic blood vessel supply area by 40%. The reduction in blood flow can be stable for 24 hours, and is accompanied by a significant decrease in the rate of wall thickening in the LAD supply area. Histology showed that there was little or no myocardial necrosis (6% of the ischemic area). The ultrastructural changes included partial loss of myofibrils and increased mitochondria and glycogen, which were consistent with the characteristics of hibernating myocardium. The fixed stenosis was removed 24 hours later, the ventricular wall motion was restored after a week, and the ultrastructure returned to normal. It was confirmed that the myocardium in the coronary artery distribution area of the stenosis was still alive after ischemia. In a later study, the laboratory found that the reduced blood flow and abnormal local wall motion caused by this LAD fixed stenosis model can remain stable for 4 weeks after surgery. As in the previous study, there was little or no myocardial necrosis (6%) in the ischemic area after 4 weeks. Another finding is that progressive myocardial apoptosis can be seen in the narrowed coronary artery supply, mainly in the subendocardial myocardium. This finding suggests that apoptosis occurs in chronic ischemic but still alive myocardium, which may lead to the loss of myocardial cells and play a role in the fibrosis of chronic hibernating myocardium. When conducting research on pro-angiogenesis therapy, attention should be paid to the process of apoptosis, because it takes a long time from causing coronary artery stenosis to giving treatment. Cardiomyocyte apoptosis may occur and cause cell loss, even if the treatment can increase the coronary artery. Blood perfusion can not get a good effect of improving local myocardial function. Regarding this point, it was also emphasized in the study of Lai et al. applying the same LAD stenosis model. These researchers found that in the 4 weeks after coronary artery stenosis, progressive left ventricular remodeling occurred, ventricular volume and mass increased, and interstitial fibrosis appeared. These changes were partially reversible after 3 weeks of reperfusion. The author believes that if there is no timely revascularization after severe vascular stenosis, local ventricular wall thinning and increased left ventricular volume will cause an increase in ventricular wall tension, which may cause progressive cardiomyocyte degeneration and fibrosis through neuroendocrine mechanisms .
Fallavollita et al. applied a similar coronary stenosis model to study. A constriction ring with a fixed inner diameter (1.5-2.25mm) was placed on the proximal end of the LAD of young pigs (average weight 8kg) and observed after 3 months. The average weight of the animals at the time of sacrifice was 75 kg. Coronary angiography after 3 months showed a high rate of complete coronary occlusion. Since the animal has not grown up when the constriction ring is placed, and the blood vessel diameter is also small, this result is expected after 3 months. . As the animal grows and the blood vessel diameter increases, it can be expected that the blood vessel stenosis where the constriction ring is placed will appear. Three months after the placement of the constriction ring, the local ventricular wall motion was significantly weakened, and the left ventricular angiography showed severe wall motion or low motion. The radioactive microsphere experiment showed that the local myocardial blood perfusion was significantly reduced (subendocardial myocardial perfusion decreased by 24%, and the whole muscle layer MBF decreased by 11%). Histological examination under light microscope did not find obvious myocardial necrosis (only 6% of the ischemic area), and myocardial contraction in the reduced function area can be restored, which also indicates that the myocardium is still alive. At the same time, local PET fluorescence deoxyglucose (FDG) imaging also showed that the myocardium in the blood supply area of LAD had FDG uptake, suggesting myocardial ischemia but still alive. Among them, FDG uptake is most obvious in the subendocardial myocardium. Coronary angiography showed that LAD with proximal occlusion can be filled with collateral circulation; the sources of collateral vessels include bridging collaterals, LCX and RCA. Animals without complete LAD occlusion did not find the formation of collateral circulation during imaging. In another study, Fallovollita et al. observed the situation at 1 and 2 months after making the same model. The average LAD stenosis at 1 month after surgery was 74%±5%, and at 2 months it was 83%. ±6%. Measurement of MBF with colored microspheres showed that the resting blood perfusion of the LAD supply range was normal at 1 month and 2 months after surgery. But in the same area, the wall motion at rest was significantly weakened, suggesting chronic stunning. The coronary blood flow reserve detected by vasodilators decreased slightly at 1 month and significantly decreased at 2 months. There was no significant change in FDG uptake at 1 month, but a significant increase at 2 months, consistent with the presence of ischemic and viable myocardium. Based on the results of the above two studies, it can be seen that the degree of stenosis and the duration of the stenosis together determine whether the stenosis coronary artery supply range can maintain normal MBF at rest. These two factors must be included in the design of pro-angiogenic treatment studies consider.
The main advantage of fixed stenosis model is that a relatively uniform degree of ischemia can be obtained in all animals. Another advantage is that myocardial blood perfusion and function in the narrow coronary artery supply range will be reduced in the resting state, which can provide an additional index for the evaluation of the effect of pro-angiogenesis treatment. The main disadvantage of this model is that due to the relatively high technical requirements of the operation, it has so far been less used in the research of therapeutic angiogenesis.
3. Hydraulic occluder model Another large animal model of chronic myocardial ischemia uses an adjustable hydraulic occluder that is placed around the epicardial coronary artery to form a fixed degree of coronary stenosis. This model also uses external pressure to cause coronary artery stenosis, but a myocardial blood flow probe is usually placed while using this device. The proximal end of the closure is in communication with the outside of the body. The closure can be expanded with gas or liquid fillings after the operation, and the degree of coronary artery stenosis can be precisely adjusted by detecting coronary blood flow. Bolukoglut is one of the first researchers to use this model to simulate hibernating myocardium. They placed the Doppler blood flow guide wire and the fluid compression narrow ring in sequence in the proximal segment of the anterior descending branch. The constriction ring is filled during the operation, reducing the blood flow rate measured by the Doppler guidewire by 50%. This degree of stenosis is maintained for 7 days. They reported that the perioperative mortality rate was as high as 44%, mostly due to malignant arrhythmia caused by coronary spasm after the expansion of the constriction ring. In the animals that survived 7 days, the systolic shortening rate in the ischemic area was reduced by 40%. There was no evidence of acidosis and cell death. The contractile function was restored after a small dose of dobutamine injection, and there was no histological manifestation of myocardial infarction. All suggest that the LAD supply range in this model is chronic ischemic but still viable myocardium. Interestingly, in the transverse section of the proximal LAD where the constriction ring is placed, damage to the adventitia and media can be seen, and subendothelial fibroblasts proliferate. This intravascular structural change may be very important and may be permanent, because even if the constriction ring is relaxed 7 days after it is filled, the local blood flow velocity remains at the level when the constriction ring is filled and cannot be restored. The same situation may exist for the aforementioned two models.
St. Louis et al. modified Bolukoglu's model and placed the ultrasound probe on the distal side of the LCX proximal fluid compression narrow ring to measure the downstream MBF. Ultrasound probes are very accurate in measuring blood flow, which can help accurately adjust the stenosis of blood vessels without causing damage to blood vessels. In this model, the placement fluid compresses the narrow ring and does not fill it immediately. The narrow ring is filled a few days after the animal recovers to avoid the impact of surgical stress on the animal. Compared with the model of Bolukoglu and others, this method greatly reduces the proportion of animals with malignant arrhythmia, only about 1%. This model has been used many times to study the pathophysiological mechanism of hibernating myocardium, pro-angiogenic therapy, and laser myocardial revascularization. After the constriction ring filling causes severe stenosis of the proximal segment of LCX, the distal arterial blood flow is reduced by 90%. After the chronic ischemic state is maintained, the transmural MBF of the myocardium in the LCX supply range measured by 13 NH3-PET is reduced by 25% to 30% . This change in blood flow level and MBF can remain basically unchanged for up to 6 months. [18F]FDG-PET also showed that the continuous FDG uptake in the LCX supply range increased by 120% to 140%, suggesting that myocardial ischemia in this range is still alive. Transesophageal ultrasonography will find that the wall motion of the LCX supply area is significantly lower at rest. After the low-dose dobutamine injection, the wall motion in this area was significantly improved compared with that at rest, and it was significantly worsened when the high-dose dobutamine was continued. This biphasic response to dobutamine is characteristic of ischemic viable myocardium. Histological examination showed that there was very little subendocardial myocardial necrosis (0%-8%) in the ischemic area. Under light microscope, it can be found that the contractile substances in myocardial cells are reduced, myofilaments are replaced by glycogen, small mitochondria are scattered in the cytoplasm, the nucleus is deformed, and heterochromatin is visible. These ultrastructural changes are all caused by chronic ischemia. Characteristics of viable myocardium. These manifestations are more pronounced in the subendocardial myocardium.
The main advantage of the hydraulic closure is that it can precisely adjust the degree of stenosis due to the assistance of ultrasonic blood flow detection, so as to form a continuous and stable ischemia. In addition, after a few days after the operation, after the animal has fully recovered from the surgical stress and the stimulating factors that cause coronary artery spasm have been removed, the constriction ring can be filled, which can more accurately determine the degree of stenosis. Another advantage is that the degree of vascular stenosis can still be adjusted after surgery, which cannot be achieved by a fixed stenosis model. Like the fixed stenosis model, this model can also cause a reduction in myocardial perfusion at rest, which facilitates the judgment of improved blood perfusion (MBF). The perioperative mortality rate of the modified model is very low. The main disadvantage of this model is the high technical requirements for surgical operations, and the placement of a constriction ring and an ultrasound probe. In long-term observation, an external part of the constriction ring will also increase the difficulty of feeding.
For various animal models, there is a common problem: how long should pro-angiogenesis treatment be performed after the ischemia. As mentioned earlier, myocardium that is still alive after chronic ischemia will undergo progressive cell apoptosis, and ventricular remodeling includes a decrease in contractile cells and an increase in interstitial connective tissue. These changes may cause the decline of ventricular function in the ischemic area. Studies have shown that if the damage of the patient's myocardial cells is more serious, the recovery of ventricular function after revascularization will be significantly affected. Such as Beanlands and other observations on patients with hibernating myocardium showed that patients with delayed revascularization had a higher rate of death before coronary artery bypass surgery, and the recovery of postoperative left ventricular function was also worse than that of patients with early surgery. Therefore, in the study of promoting angiogenesis, the time of chronic myocardial ischemia in animals should not be too long. Otherwise, even after the local myocardial blood flow is restored, myocardial function will be difficult to recover, which will affect the effect of the pro-angiogenic substance. Evaluation.
A variety of pro-angiogenic treatments have been proven effective in different porcine chronic myocardial ischemia models, including recombinant FGF and VEGF proteins, gene therapy, autologous bone marrow cell transplantation, and laser myocardial perforation, but they are far from the same in clinical trials. The desired result. The reason for such a big difference between experimental research and clinical research is not clear, but it may be related to the difference between model animals and humans. Until recently, the formation of new blood vessels during chronic ischemia has been considered to include the processes of arteriogenesis and angiogenesis. Angiogenesis refers to the formation of new capillaries in the original capillary bed by sprouting, which is mainly caused by ischemia. The activation of HIF promotes the expression of VEGF and its receptors and improves the stability of VEGF mRNA. The formation of arterioles is the growth of new arterioles on the basis of the original arterioles. This vascular growth is the process of collateral vessels maturing and forming blood vessels that can pass blood. The formed vessels can be seen during angiography. The stimulating factors for the formation of small arteries include vascular shear and inflammation. At this time, the infiltration of monocytes and other white blood cells leads to the production of growth factors such as FGF and promotes the growth of blood vessels. Recent studies have shown that in addition to the above two processes, adult neovascularization also has a process of vasculogenesis. Angiogenesis refers to the process of forming vascular tissue from a kind of endothelial precursor cells, angioblasts. Angioblasts migrate and fuse with other endothelial precursor cells and capillaries to form a primitive vascular network, also known as primary capillary plexus. Subsequently, the primary capillary plexus undergoes sprouting and branching remodeling, which is the process of angiogenesis. Therefore, although some authors believe that arteriogenesis is necessary for the obvious restoration of myocardial blood flow and the therapeutic effect of angiogenesis, angiogenesis, angiogenesis, and arteriogenesis may all participate in the process of adult cardiac neovascularization. Most of the research on arteriogenesis is carried out in areas with abundant original collateral vessels such as limbs. Does the pro-angiogenesis treatment in the heart also require the participation of original collateral vessels? Or does it depend on the true meaning? The formation of new collateral vessels? It is very important that the formation of small arteries is based on the original small arteries. In patients with severe coronary heart disease, the corresponding foundation may be lacking. In these patients, the process of angiogenesis and angiogenesis may also be impaired.
In animal experimental research, healthy animals are often used to cause single-vessel disease. In this case, the ability to form new collateral vessels may be relatively strong, which is different from the situation of clinical patients. This may be caused by the effect of clinical trials. The reason for the great difference in animal experiment results. In addition, it may be necessary to establish an animal model of multivessel disease, and more rigorously evaluate the methods that may be effective in the single-vessel disease model. At the same time, neovascularization is a highly ordered physiological process regulated by a variety of active molecules, such as soluble protein molecules VEGF, angiopoietin, FGF, PDGF, TGF, INF, CSF, etc.; some membrane-bound proteins also play an important role The effect of; the shearing force on the surface of endothelial cells also has a regulatory effect on capillary formation. Full attention and in-depth research should also be given to the interaction of these regulatory factors and their effects on treatment. In the research of angiogenesis, genomics and proteomics research will also occupy a more important position. In such studies, using mice or rats as model animals may have more advantages.