
Groundhog (marmotamonax, English name Groundhog) is also called groundhog. The average weight of a groundhog is 4.5kg, the largest is 6.5kg, and the length is about 56cm. Land squirrels are mainly distributed in the Great Plains of North America in Canada and other regions, and belong to the rodent family like squirrels, beavers and chipmunks. Animal hepatitis B viruses include woodchuck hepatitis virus (WHV that only infects woodchucks in the eastern northeastern United States), ground squirrel hepatitis virus (GSHV), and arctic squirrel hepatitis virus (ASHV). Sequencing showed that the nucleotide and amino acid sequence differences between WHV and GSHV viruses are very small, and the degree of variation is not greater than the sequence variability between human HBV subtypes. The Woodchuck model is also the most widely used model in HBV research, because the genome organization of WHV and HBV is more similar than that of avian hepatitis virus, so the entire virus life cycle can be studied in the natural host. The woodchuck hepatitis virus is a virus that spontaneously infects the eastern woodchuck.
The virus was first discovered in Perros Park in Philadelphia in 1977 and can cause chronic hepatitis and liver cancer in Woodchuck. .. At present, several WHV virus strains have been isolated, their genotypes are relatively close, but the infection of neonatal woodchucks can cause chronic hepatitis in varying degrees. The Woodchuck hepatitis model is one of the most widely used models in the study of HBV infection and antiviral therapy.
Cornell University promoted the Marmot team to a laboratory model in 1980. The initial model research progress includes: experimentally infected adult and juvenile woodchucks are undergoing virological and spontaneous research on liver cancer. Since 1988, the new Woodchuck WHV model has been used to detect nucleotide analogs against viral infections.

Early studies also tested traditional antiviral vaccines against acute and self-limiting WHV infections in adult and newborn groundhogs, and used groundhog models to immunize newly immunized woodchucks and tested the vaccine against chronic hepatitis And the preventive effect of liver cancer. Use the immunosuppressant cyclosporin A to study the immune regulation of acute and chronic infections and determine the role of the host immune system in the self-limitation and chronic transformation of WHV infection. Since the 1980s, research on woodchuck hepatitis virus has focused on the establishment and application of models, including the pathology of viral infections and diseases, antiviral drug screening, and individual or systemic immune regulation.
It is mainly as follows:
(1) Natural incidence of WHV infection and disease: The woodchuck hepatitis virus model is a model widely accepted by researchers studying hepatitis B virus infection. Recent studies on the response of woodchucks to WHV infection revealed a series of immune mechanisms of WHV infection. The immune markers of woodchuck virus infection are not exactly the same as those of humans and mice. However, in the process of woodchuck infection, the type and intensity of the immune response is very similar to the process of human hepatitis B virus infection. Infecting adult or newborn woodchucks with WHV will make the corresponding acute infection become self-limited infection and develop into two types of chronic infection, which is related to the impact of human age on the consequences of HBV infection. I was working as an adult Chuck infected with WHV, most people will heal on their own, and about 5% of people will develop chronic hepatitis.
However, during viral vaccination and acute infection of adult woodchucks, because cyclosporin A temporarily suppresses cell-mediated immunity, 92% of infections can develop into chronic carriers. Cyclosporin A is injected only during the vaccination period and the early stage of acute infection (0-4 weeks), and 50% of infections develop in chronic carriers.
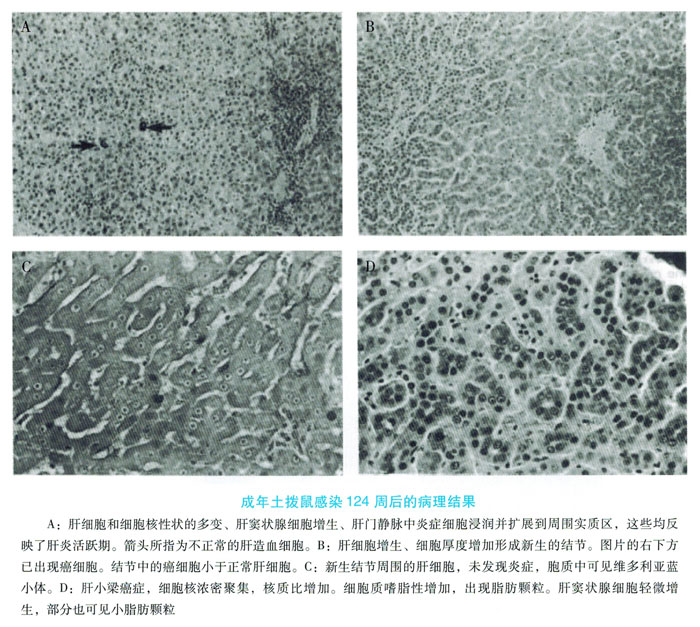
Most chronic WHV infections are caused by neonatal acquired infections. Neonatal woodchuck virus infection can develop into a chronic carrier at a rate of 60% to 75%, of which 25% to 40% can recover spontaneously. When prairie dogs are infected in the spring, the viral load is consistent with the host's immune response, so the samples collected in the second year can be used for data modeling to analyze serological and liver changes. These features also allow the model to be used to simultaneously detect host responses during acute infections before self-limitation, as well as serological features after conversion to chronic infections. Therefore, it is possible to identify and distinguish the mechanisms that cause self-limiting and chronic infections.
Chronic WHV infection can cause the virus to replicate for a long time, leading to chronic liver disease and liver cancer. Woodchuck with chronic WHV infection will not spontaneously transfer from e antigen to e antibody in serum, and gradually reduce virus replication. This is more common in human hepatitis B infections. Generally speaking, chronic WHV carries a large amount of virus replication in woodchucks, and a very high surface and e antigen load is essential for maintaining the host's immune tolerance from chronic liver disease to liver cancer. Yes. Self-limiting WHV infection can basically inhibit virus replication and completely eliminate the virus in the body. However, in self-repairing woodchucks, trace amounts of WHV genome were detected in the liver, serum, and peripheral monocytes, which may be another persistent infection. Similarly, people who healed after being infected with HBV can detect traces of HBV DNA. Compared with seronegative (not infected with WHV) woodchucks, woodchucks can heal themselves after infection, but there is still a 5-20% chance of developing liver cancer. However, compared with long-term infected groundhogs, this possibility is very low (the latter is 100%). The results can compare chronic WHV and chronic HBV patients and liver cancer caused by liver cancer.
Recently, some scholars have used CsA to suppress the immune system's response to woodchucks infected with WHV, study the activation of WHV virus replication, and study wood with WHV DNA or virus antigens. We found that Chuck remained in the liver and peripheral blood. Short-term recovery of WHV can be seen during CsA treatment. This may be because the virus is suppressed by the host's virus-specific immune response and maintains a low level of replication. The interaction between the long-term memory of the host cell immune response and the low level of virus replication is very important for long-term stable control of virus replication and suppression of the infection process, thereby maintaining the recovery period. For antiviral or immunotherapy of chronic HBV or WHV infection, please refer to the recovery period immune status of spontaneous infection.
(2) Virus molecular biology research: WHV belongs to the family of hepatitis and primary hepatitis. The genetic composition of WHV is similar to HBV and other mammalian liver viruses, and has basically the same biological characteristics and replication strategies. In the serum of woodchuck infected with WHV, the envelope protein (WHsAg surface antigen) constitutes linear or spherical particles, and the diameter of the complete virus particle is 42-45m. The virus contains a nucleoprotein or core protein (WHcAg) and a DNA genome. The replication cycle of WHV and HBV is the same. In some studies, the WHV genome was cloned, digested, and circularized into supercoiled DNA, and then injected into the liver of parenchymal woodchucks to cause WHV infection. The mechanism of hepatitis B virus invading liver cells is not clear. Studies have shown that certain sequences in the S1 pre-region of the large envelope protein may be responsible for all or part of the mutual recognition of viruses and cells, but so far the liver cell receptor has not been fully identified. However, in vivo neutralization studies using monoclonal antibodies have increasingly revealed the recognition sites of other parts of the envelope protein, and vaccines prepared from small envelope proteins alone can exert effective protection. After the virus enters the cell, part of the double-stranded circular DNA enters the nucleus, and is converted into complete double-stranded DNA by the endogenous reverse transcription DNA polymerase connected to the virus particle, thereby forming a complete double-stranded DNA. It is a covalently closed circular cccDNA. Under the action of RNA polymerase II in the cell, cccDNA acts as a template for viral mRNA transcription. The mRNA transcript (slightly larger than the viral genome) is integrated into the viral polymerase and mature core antigen particles, and the RNA-dependent DNA polymerase activity of the viral polymerase can catalyze negative transcription.
Then, the virus uses its own DNA-dependent DNA polymerase activity to synthesize about 50% to 75% of the length of the negative strand of positive strand DNA. This unclosed circular double-stranded DNA is found in mature WHV and HBV virus particles. The envelope protein is synthesized in the endoplasmic reticulum of the cell, and mature virus particles are released from the liver cells. The hepatitis B virus itself is not toxic to infected cells. In HBV, most core antigen immunostaining is found in the nucleus, while in WHV, most core antigen staining is found in the cytoplasm and not in the nucleus. This indicates that the newly synthesized cytoplasmic core antigen is responsible for reverse transcription, synthesis of part or all of the positive strand DNA, and occasionally reenters the nucleus to synthesize cccDNA. In HBV, the core antigen in the cytoplasm is lower than the detection level (immunostaining), and the dense staining of nuclear antigen indicates that the core antigen is now mature. Then, the mature core antigen reaches the endoplasmic reticulum through another cellular pathway to assemble the surface antigen. In the established Woodchuck chronic WHV infection model, compared with chronic carriers of human HBV, the concentration of WHV particles is 10 to 100 times that of the latter. This may be related to the different immunolocalization of the core antigen between the two. In the WHV replication cycle, the DNA-to-RNA transcription process is similar to retrovirus, but the DNA is not integrated into the host's genome. The free cccDNA in infected liver cells is stable, and the infected cells need to be removed from the system, which makes it one of the main targets for the removal of the host hepatitis B virus. After the hepatitis B virus DNA enters the cell genome, it must be truncated and rearranged, and it can be inserted into any position in the cell genome, which is essential for the transformation of liver cells. Morphological and virological studies of the liver found that in woodchucks infected with WHV, 100% of the liver cells were infected with the virus. Although the viral intermediates are removed when the acute infection subsides, cccDNA still exists for a long time after viral replication ceases.
(3) Immunological research: After newborns and adult woodchucks are infected with WHV, their recovery requires a specific immune response to the virus in the liver and the surrounding environment to eliminate the virus. The natural recovery process is the benchmark for antiviral or immunotherapy for chronic infections. Many studies have shown that inducing specific cellular and humoral immune responses is a prerequisite for adult patients to clear HBV. In most cases, compared with human patients, there are no obvious clinical symptoms after HBV infection. Therefore, the development of adult HBV incubation period and acute period immune response and its effect on infection and its role in eliminating infection have not been studied in detail. Although hepatitis B virus is generally considered to be hepatitis B virus, hepatitis B virus DNA can be detected in tissues other than the liver. DHBV can replicate in duck pancreas. HBV and WHV can infect the lymphatic system. This phenomenon is currently unknown. Some studies have shown that WHV replicates and spreads in lymphatic tissues even before it infects the liver. Using lipopolysaccharide to stimulate peripheral blood mononuclear cells of woodchucks carrying chronic WHV can activate static DNA and form replication intermediates. Lipopolysaccharide stimulates the cell-free supernatant of peripheral blood mononuclear cells of woodchuck. These supernatants carry chronic WHV and can infect sensitive adult woodchuck and cause acute infection. Whether the WHV genome of lymphatic tissue is in a static or replicated state depends on the state of host lymphocytes, including dormancy, division, circulation in blood and lymphatic tissues. One month after the newborn woodchuck was infected with WHV, WHV DNA was detected in the bone marrow, but during the acute replication stage, early replication markers of WHV appeared in peripheral blood mononuclear cells, lymph nodes and spleen. During recovery after infection, WHV replication in lymphoid tissues tends to be stable (or completely eliminated). In chronic infection, WHV replication of peripheral blood mononuclear cells is also quiescent, but usually replicates in the spleen, and the quiescent WHV of peripheral blood mononuclear cells can be activated in vitro by lipopolysaccharide stimulation. I will. More and more studies have also shown that the peripheral blood mononuclear cells of woodchucks recovered from acute infection carry infectious WHV DNA. In short, whether it is recovered from an acute infection period or a chronically infected woodchuck, the WHV infection status of peripheral blood mononuclear cells is similar. In the above two cases, ConA, PHA and LPS are used. When stimulated by mitogens, peripheral blood mononuclear cells increased significantly. Infection of the lymphatic system does not affect the immune response triggered by the virus. in fact. Regardless of acute or chronic WHV infection, there is no opportunistic infection caused by lymphadenopathy, lymphopenia, lymphoma and systemic immune deficiency. As with the consequences of natural recovery, treatment of chronic WHV should focus on all viral reservoirs and various forms of replication in lymphoid tissues and liver. One item of self-limiting WHV
Research has shown various types of immunity related to self-limiting human HBV infection. Generally speaking, the self-limited infection of neonates and adult woodchucks has the following characteristics: (1) During the acute infection period, viral DNA and antigen peaks are detected in the liver and serum; (2) rapid cells against antigens Immune response; ③Acute viral hepatitis but mild liver injury; ④Short peak of transaminase activity in the serum, which then returns to normal; ⑤Virus neutralizing antibodies appear in the serum, causing viruses and viral antigens in the blood and liver on sunny days. Humoral immunity to viral antigens during the recovery process has the following characteristics: high titers of anti-core antigen antibodies are produced during the onset and reduction of acute infection (usually within a few weeks after laboratory infection). And anti-surface antigen antibodies with neutralizing and protective activities. In adult woodchucks, cell-mediated immunity involves the activation of peripheral blood mononuclear cells. This has been confirmed by stimulating peripheral blood mononuclear cells in vitro with WHsAg, WHcAg and synthetic peptides of the two antigens. The successful response of peripheral blood mononuclear cells to WHcAg is related to the virus clearance rate in serum, and virus clearance is related to several important antigenic sites of WHcAg. Compared with unimmunized woodchucks, the main epitope region (97-110 amino acids) of WHcAg antigen is used to immunize adult woodchucks. WHV infection weakens the acute infection response. During the recovery from acute infection, the response of peripheral blood to WHsAg and WHXAg is still being studied. In the acute phase of WHV infection in newborn woodchucks, no matter what virus inoculation is used, their cellular immune response is similar to that of adult woodchucks. The response of healthy peripheral blood mononuclear cells to viral antigens is essential for removing viral DNA and WHsAg in serum. A detailed study of the immune response caused by WHcAg revealed several specific epitopes of the antigen. Similar to adult woodchuck infections, newborn woodchucks also react with the main area of the WHcAg antigen (amino acids 97-110, amino acids 100-113).
In the liver, the self-limiting features of adult autophagic Woodchuck infection include mild to significant liver inflammation and damage, CD3 + T cell accumulation, and liver cell apoptosis and regeneration.
(4) Antiviral drugs: The study of woodchuck hepatitis B model provides a variety of factors that can affect viral infection, replication and chronic infection. Because WHV and HBV DNA polymerase have similar biochemical properties, the Woodchuck model can be used to evaluate antiviral drugs. Using this model, scholars are evaluating numerous nucleotide analog drugs and other antiviral compounds. In the Woodchuck model, we found that after 6 weeks of antiviral non-aryl therapy, the drug has long-term toxicity, resulting in insufficient liver function and lactic acidosis. This symptom mimics the fatal side effects caused by the clinical use of non-aromatic therapy, indicating that woodchucks can be used for preclinical evaluation of hepatitis B antiviral drugs. However, in large-scale drug screening, groundhogs are difficult to operate and expensive, and it is still difficult to overcome their own shortcomings that are difficult to spread.
The virus of the hepatitis family is very popular in pigs and is called porcine hepatitis B virus (SHBV). Hepatitis B clinical test kit (ELISA kit), immunohistochemistry and electron microscope can detect SHBV. In the detection of SHBV-infected pigs, 24.8% can detect HBsAg and anti-HBsAg, but e antigen and e antigen antibody can hardly be detected. Detection of surface and core antigens in liver samples by immunohistochemistry. Cells in the liver parenchymal area experience punctate deformities and necrosis, resulting in a sharp increase in fibrous connective tissue. Like HBV particles, transmission electron microscopy can be seen in the nuclear area of liver cells, divided into 20m and 40m particles, corresponding to HBV spherical and Dane particles, respectively.
As a laboratory animal, pigs have unparalleled advantages over other animals that are susceptible to HBV. Pigs are widely used in medical research and have a good source of supply. Therefore, detailed studies on porcine hepatitis B models will help promote the etiology and immunology of HBV infection, and are expected to become more common HBV model animals in the future.