
When it comes to respiratory "killers", many people first think of lung cancer. However, there is a "cancer-like disease that isn't cancer" — pulmonary fibrosis (PF), which is no less dangerous than lung cancer. Among them, idiopathic pulmonary fibrosis (IPF) has an average survival time of only 3-5 years from diagnosis to death.
What is pulmonary fibrosis (PF)?
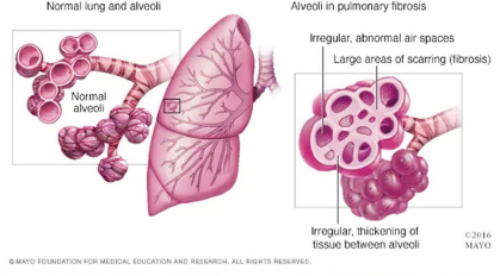
Image sourced from the internet
Pulmonary fibrosis (PF) is a lung disease characterized by chronic inflammation and excessive fibrosis of the pulmonary interstitium and alveolar walls. Its main feature is significant changes in the structure, composition, and stiffness of lung tissue, leading to the deterioration of lung function.
PF is divided into idiopathic pulmonary fibrosis (IPF) and secondary pulmonary fibrosis (secondary PF). Among them, the pathogenic factors of secondary PF mainly include (1) environmental factors such as dust exposure, (2) medication-related factors such as the chemotherapeutic drug bleomycin, and (3) disease-related factors such as chronic obstructive pulmonary disease (COPD); while IPF may be associated with factors such as aging, abnormal epigenetic regulation, intestinal microbiota dysbiosis, and smoking.
From an epidemiological perspective, the global prevalence of IPF is approximately 13-20 per 100,000 people, while the incidence rate of IPF in China is about 1-5 per 100,000 people. Notably, with the acceleration of population aging, increased exposure to environmental pollutants, and other contributing factors, the incidence of pulmonary fibrosis has been showing an annual upward trend, imposing an increasingly heavy disease burden on patients' families and society.
Advances in Multidimensional Innovative Therapies for Pulmonary Fibrosis (PF)
Currently, pulmonary fibrosis remains incurable in clinical practice and requires long-term intervention. Clinically, it is mainly treated with drugs such as Pirfenidone and Nintedanib; however, these drugs can only delay the decline in lung function and cannot reverse pulmonary fibrotic scars. Additionally, they may cause intolerable side effects such as gastrointestinal discomfort, resulting in a lack of diverse clinical treatment options, limited choices for patients, and inadequate therapeutic efficacy, with a prominent unmet clinical need.
In 2025, this stagnant landscape was completely shattered as innovative breakthroughs across multiple disciplines and technologies emerged collectively, and the treatment landscape of pulmonary fibrosis underwent a transformative leap forward.
1. First-in-Class Drug for Pulmonary Fibrosis Approved First in China, Ending a Decade-Long Drought of New Treatments
The year 2025 has witnessed a global upsurge in innovations for pulmonary fibrosis treatment. On October 7, the U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim’s breakthrough therapy Boyouwei (Namilust) for the indication of idiopathic pulmonary fibrosis (IPF) in adults. Shortly thereafter, China’s National Medical Products Administration (NMPA) expeditiously granted approval for the drug’s IPF treatment on October 22. Merely seven weeks later, on December 10, China took the lead again by approving it for the treatment of progressive pulmonary fibrosis (PPF) in adults. For both core indications of IPF and PPF, China’s approvals preceded those in Europe, the United States, Japan, and other regions.
Namilust is a small-molecule phosphodiesterase 4B (PDE4B) inhibitor that selectively targets the PDE4B isoenzyme. As the first IPF therapeutic drug in a decade to meet the primary endpoint in Phase III clinical trials and secure approval, the launch of Boyouwei (Namilust) has broken the deadlock of no new drugs approved for pulmonary fibrosis in over 10 years, marking that the clinical treatment of idiopathic pulmonary fibrosis has entered a new breakthrough phase.

Image sourced from FDA

Image sourced from Boehringer Ingelheim
2. AI-Powered Target Innovation: The World’s First AI-Discovered Drug Demonstrates Prominent Clinical Value
In August 2025, the results of the Phase IIa clinical trial for idiopathic pulmonary fibrosis (IPF), led by the research team of Xu Zuojun from Peking Union Medical College Hospital, were published in Nature Medicine. As the world’s first small-molecule TNIK inhibitor whose R&D and design were fully guided by artificial intelligence (AI), rentosertib demonstrated impressive clinical outcomes: in the 60 mg cohort, forced vital capacity (FVC) increased by 98.4 mL from baseline, in stark contrast to a 20.3 mL decrease observed in the placebo group. Safety data indicated that adverse events (AEs) were predominantly mild to moderate, with good tolerability overall. This agent offers a new glimmer of hope for patients by "reversing impaired lung function" and has already been granted orphan drug designation by the U.S. Food and Drug Administration (FDA).
It should be noted that the trial enrolled only 71 patients with a 12-week follow-up period, which presents limitations of a "small sample size plus short observation duration". This breakthrough not only marks China’s leap from being a follower to a leader in AI-driven original new drug development, but also provides a Chinese solution for the global treatment of rare diseases.
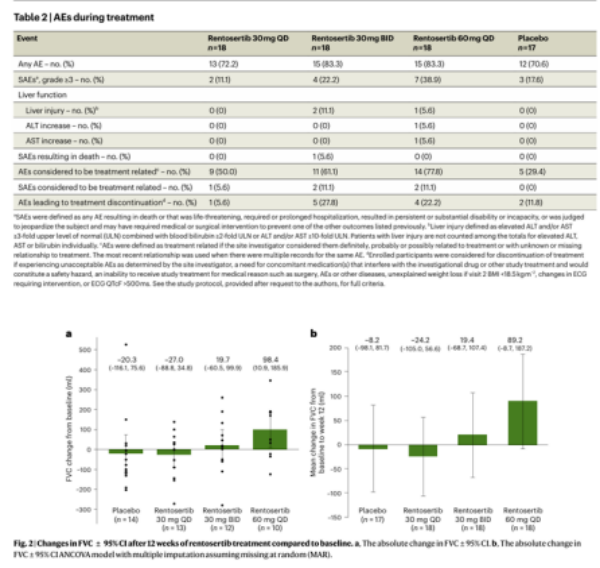
Image sourced from Nature Medicine
3.A New Direction for Stem Cell Therapy: Unlocking the Full Potential of Treating Refractory IPF
In February 2025, a team of Chinese scientists published a landmark research finding in eBioMedicine, a sister journal of The Lancet. The study achieved three core innovations that broke through the bottlenecks in stem cell therapy for pulmonary fibrosis: for the first time globally, it systematically clarified the core application potential of human small bronchial epithelial stem cells in the treatment of refractory idiopathic pulmonary fibrosis (IPF). Additionally, the innovative regimen of "autologous basal stem cells plus minimally invasive bronchoscopic infusion" opened up an entirely new therapeutic pathway for end-stage patients.
After receiving treatment, three patients with severe pulmonary fibrosis showed significant improvements in forced vital capacity (FVC) and small airway function indicators (FEF75, FEF50). High-resolution computed tomography (HRCT) scans revealed increased lung volume and reduced fibrotic areas. The patients also experienced marked relief of subjective symptoms such as dyspnea, along with substantial enhancement in quality of life. This research provides robust clinical evidence for the translational application of stem cell therapy in the field of refractory pulmonary fibrosis.
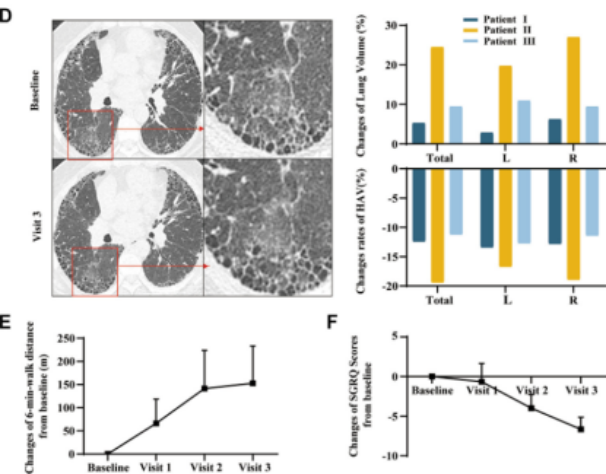
Image sourced from eBioMedicine
Summary
In 2025, the field of drug research and development (R&D) and clinical treatment for pulmonary fibrosis (PF) has demonstrated distinct developmental characteristics marked by "multi-mechanism exploration, multi-stage intervention, and multi-technology integration." Previously, treatment was confined to the singular goal of "delaying disease progression." Today, with continuous advancements in innovative research, therapeutic strategies for PF are steadily shifting toward earlier precise intervention, more individualized precision treatment, and more effective fibrosis reversal. It is believed that as these innovative research outcomes continue to be translated and implemented in clinical practice, the future will bring more diverse, safer, and more effective treatment options for PF patients. Moreover, this once life-threatening disease, long regarded as "incurable," is now seeing the dawn of a cure and renewed hope.
Zvast Bio Pulmonary Fibrosis Model
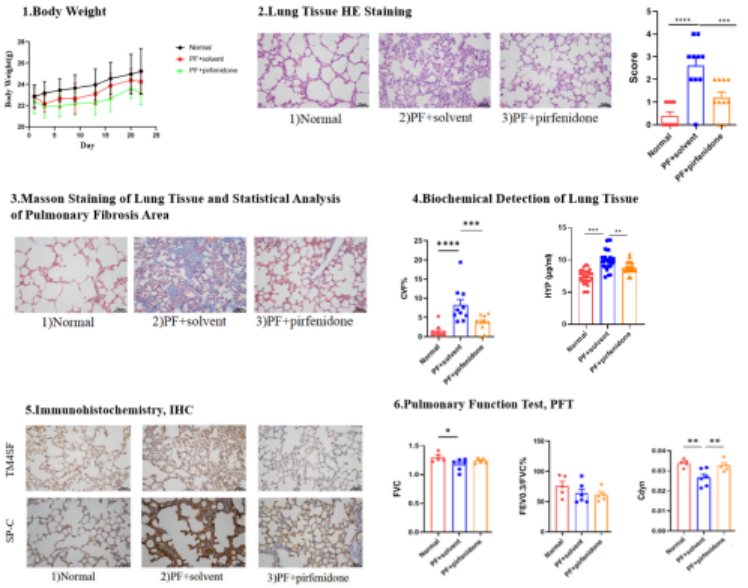
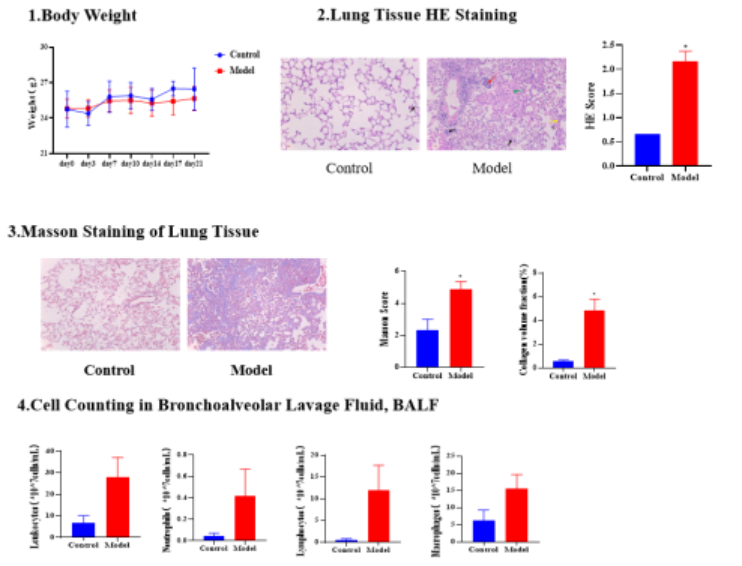
1.Bleomycin-Induced Mice Pulmonary Fibrosis Model
Animals: 7-week-old C57BL/6 mice (half male, half female)
Modeling: After cutting the neck skin, bluntly dissecting subcutaneous muscles to expose the trachea, bleomycin solution was slowly injected into the trachea. Mice in the control group received an equal volume of 0.9% normal saline via intratracheal injection.
Positive Drug: Pirfenidone capsules (administered by gavage)
Results:
2.Bleomycin-Induced Mice Pulmonary Fibrosis Model
Animals: 22-week-old female C57BL/6 humanized mice
Modeling: After cutting the neck skin, bluntly dissecting subcutaneous muscles to expose the trachea, bleomycin solution was slowly injected into the trachea. Mice in the control group received an equal volume of 0.9% normal saline via intratracheal injection.
Positive Drug: Pirfenidone capsules (administered by gavage)
Results:
References:
1.Xu Z.A generative AI-discovered TNIK inhibitor for idiopathic pulmonary fibrosis: a randomized phase 2a trial. Nat Med. 2025 Aug;31(8):2602-2610.
2.Liu Z. Epithelial stem cells from human small bronchi offer a potential for therapy of idiopathic pulmonary fibrosis. EBioMedicine. 2025 Feb;112:105538.